With price pressures increasing and tight supply, the temptation grows to rely on diverted or gray market products. But these products are outside our control and could include counterfeits. “If we could only trust the counterfeiters to follow GMP and conduct a recall if they find a problem.”
[NOTE: Definitions: Gray Market (Black’s law): A market in which the seller uses legal but sometimes unethical methods to avoid a manufacturer’s distribution chain and thereby sell goods (especially imported goods) at prices lower than those envisions by the manufacturer. Diverted good or Product Diversion (FFPA): “refer to products that are sold outside of the intended market or distribution channels, often without the knowledge or permission of the manufacturer.” Often, the lack of transparency enables counterfeit product leaking into the legitimate supply chain.]
When “Just Cheaper” Turns Criminal
During a traffic stop in August 2021, Michigan State Police in Muskegon found Demario Wilson in possession of more than 15,000 fake pills labeled as Adderall—but laboratory tests revealed they were actually methamphetamine [DOJ, 2022]. That’s 15,000 opportunities for someone to unknowingly ingest a powerful and dangerous drug. Just imagine: 15,000 people thinking they’re taking a genuine prescription-quality stimulant when in fact, they’re ingesting “meth” sourced from an unregulated and criminal operation.
Diverted and Gray Market Goods – The Thin Line Between Cheaper and Catastrophic
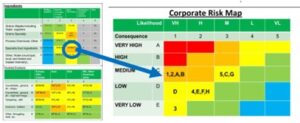
This case highlights the risks associated with diverted and gray market goods. These products often appear genuine, but the lack of transparency creates a vulnerability for counterfeit product to enter the legitimate supply chain. Of course, the counterfeiters would lie and say this is “genuine, diverted product.” The counterfeit product labeling is near perfect, and even experts may require forensic testing to detect counterfeits (Figure below). The problem is that “nine times out of ten, the product is just fine”—a tax-avoidance smuggling or a generic clone with the same active ingredient. But that tenth time could be catastrophic.

That’s the point. The product might seem “safe enough,” and is often genuine product that is just shipped through a non-traditional channel, and this creates a comfort level. However, unlike regulated supply chains, gray market sources often lack transparency, traceability, and compliance with Good Manufacturing Practices (GMPs). There’s often no oversight, no recall process, no batch records, and certainly no customer service. If the pills are fake and laced with methamphetamine or Fentanyl, the counterfeit manufacturer does not take responsibility.
The diverted or gray market product – whether it is counterfeit or not – often has no traceability back to the source. The lack of traceability leads to the inability – or at least extreme difficulty – in conducting a recall.
Has anyone ever heard of an illegal drug dealer offering a refund to their customers due to a quality control concern? Also, if a buyer feels cheated, they would logically be very hesitant to complain to law enforcement (“My meth dealer cheated me, so I want you to go arrest them.”).
“Just One Xanax”—The Deceptive Calm Before the Overdose
A recent Michigan State Policy/Drug Recognitions Expert conference presented how front-line law enforcement identifies behavioral indicators during traffic stops. One anecdote was particularly telling. A driver was pulled over for erratic behavior and was determined to have the equivalent of one beer and a single Xanax that was bought online. The reality? The Xanax had been spiked with Fentanyl.
The Drug Recognition Expert on the scene noted fentanyl opioid-like symptoms inconsistent with what would be expected from a drug like Xanax. The explanation became clear when toxicology reports came back: it was Fentanyl. Why spike counterfeit pills with Fentanyl? Because it’s cheap, addictive, and in small doses, it gives the “kick” counterfeiters think customers want—until it doesn’t, and the dose becomes lethal.
Application to the Food Supply Chain: What if This Were Your Product?
In the food industry, diverted and gray market products follow a similar risk profile. Perhaps it’s surplus inventory from another market or a reformulated version not intended cleared for your geography. You might be told, “It’s the same stuff, just cheaper.” And it probably is—until it’s not.
If we could trust the counterfeiters to follow GMPs (good manufacturing practices), verify authenticity, and conduct recalls (we cannot), then maybe diverted goods would be a risk worth taking. But they don’t, and we can’t. That’s why these risks lie outside the food safety or food fraud risk tolerance threshold. The only way to bring diverted or gray market good within tolerance is to implement effective countermeasures and controls such as extensive authenticity tests if they are available.
Take a Hard Look at Your Supply Chain
Let’s be clear: This is not just about pharmaceuticals, illicit drugs like fentanyl or cocaine, or isolated events. This is about understanding the blind spots in your supply chain. You may never see a fake Adderall tablet, but what about mislabeled spices, counterfeit supplements, or diluted olive oil? Each product has its own gray market—and each presents unique vulnerabilities.
Tools such as Food Fraud Vulnerability Assessments (VACCP) and the Food Fraud Suspicious Activity Report (FFSAR) provide structured, methodical ways to evaluate risk. But they only work if you use them. Following a methodical approach to assessing vulnerabilities can help keep your supply chain within the “risk tolerance.”
For more information and resources on conducting Food Fraud Vulnerability Assessments, see: Food Fraud Vulnerability Assessment & Prevention Strategy (VACCP) MOOC
Takeaways Points
• Diverted or gray market products may appear safe and cost-effective. Still, without scrutiny, they can expose critical vulnerabilities and must be brought within acceptable risk tolerance.
• Nine out of ten times, gray market purchases may seem harmless—but that tenth time can cause catastrophic consequences.
• Always begin with a food fraud vulnerability “initial screening”—many risks are manageable with simple countermeasures that significantly reduce exposure and improve supply chain resilience.
[Note: The GFSI food safety management system REQUIRES a review of diverted or gray market goods. See the GFSI Food Fraud Technical Document (LINK: https://mygfsi.com/wp-content/uploads/2019/09/Food-Fraud-GFSI-Technical-Document.pdf).]
Reference:
[Note: Supported by ChatGPT]